Long-Term Treatment Success - results powered by ENTYVIO.
Treatment success in UC isn’t just about short-term remission - it’s about sustained outcomes that minimize relapse, reduce steroid dependence, persistence. Entyvio offers proven durability and safety, making it a cornerstone for long-term disease control.
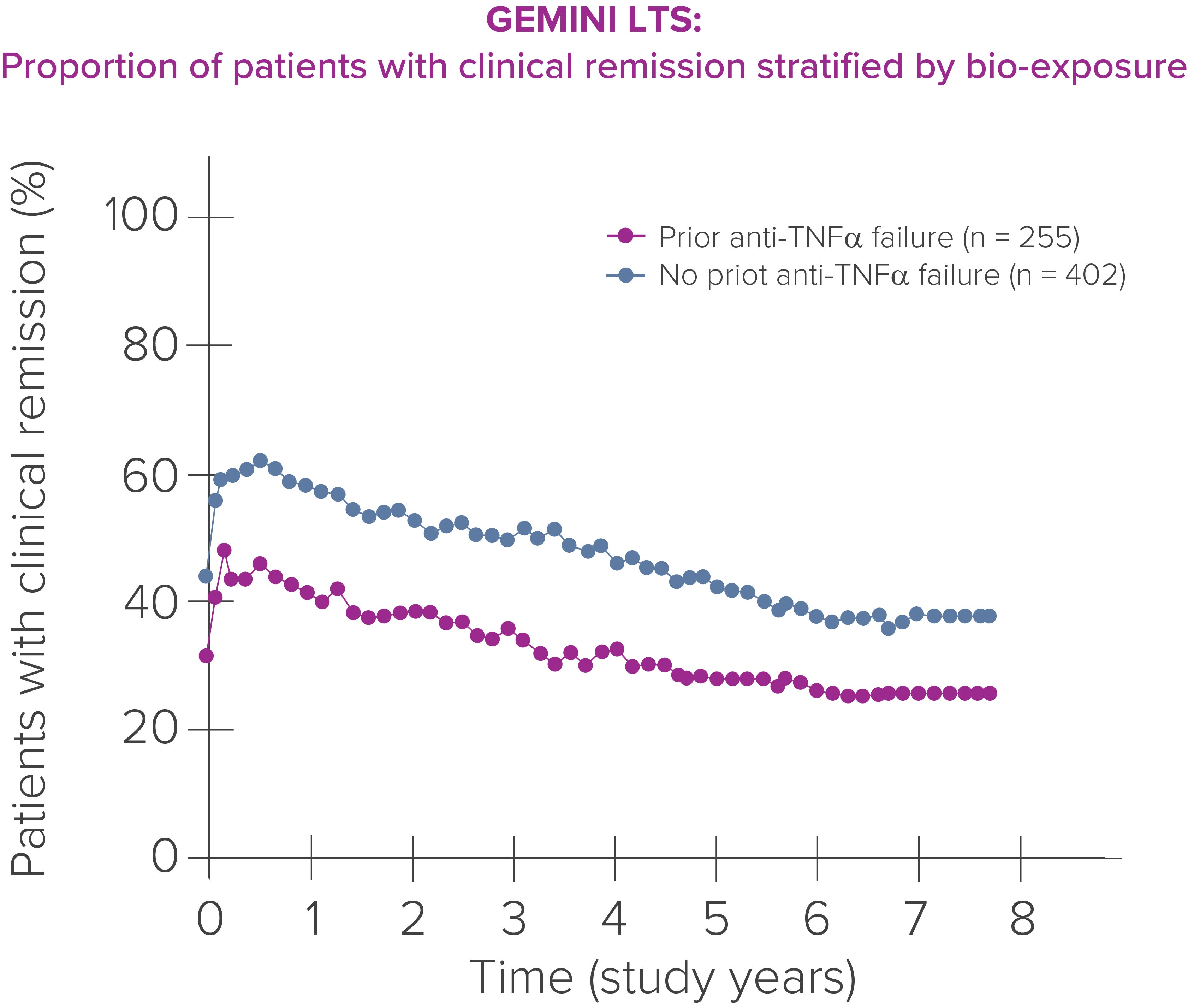
In GEMINI LTS, bio-naïve patients with UC demonstrated higher rates of clinical response and clinical remission compared to those who had previously experienced TNF-antagonist failure. [1]
Clinical remission defined by a partial Mayo score of ≤2 with no individual subscore of >1.
LTS, Long-term Safety; TNF, tumor necrosis factor; UC, ulcerative colitis
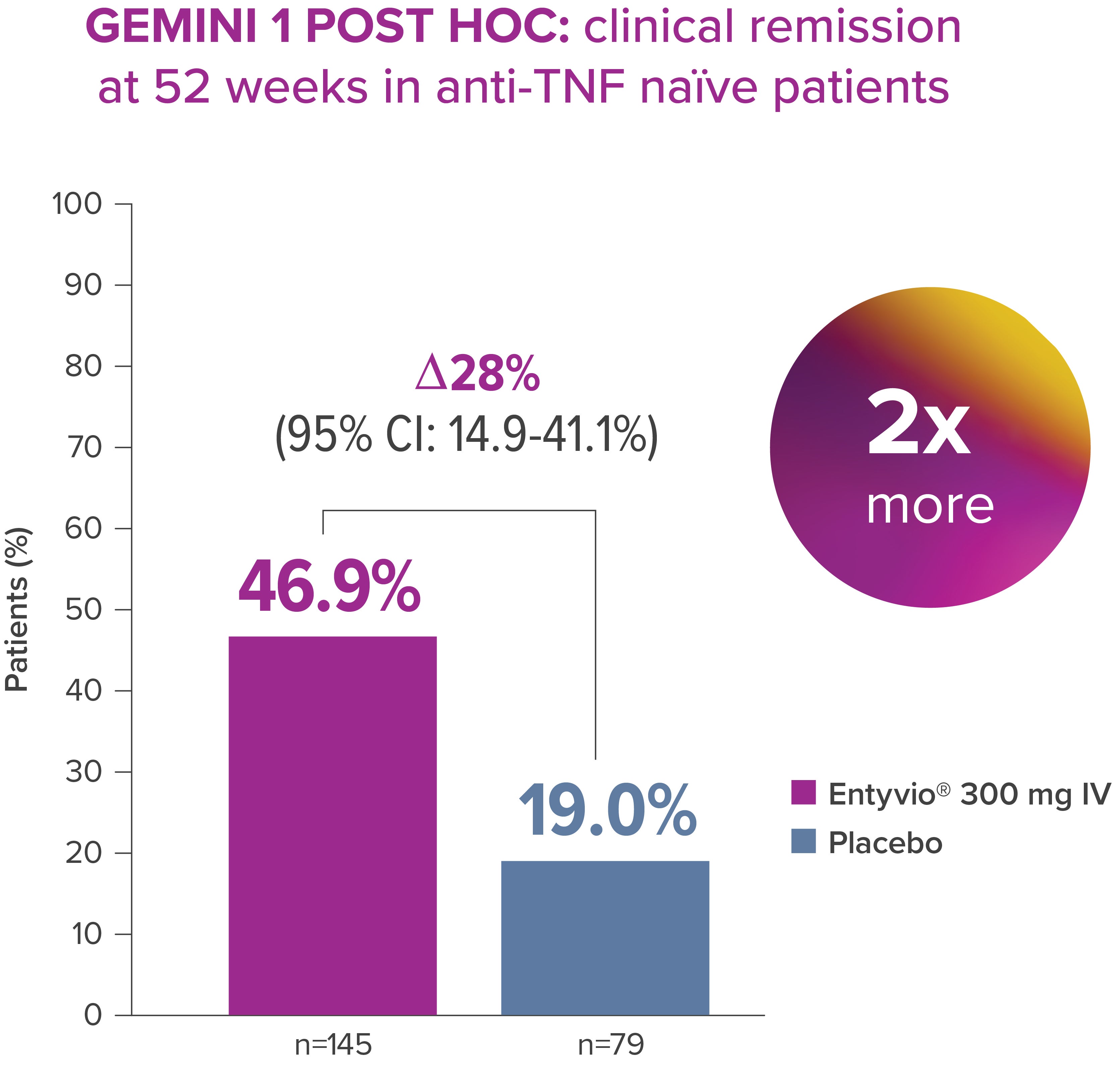
Entyvio® can offer improved long-term clinical remission* compared with placebo [2]
In a post hoc analysis of GEMINI 1, more than twice as many anti-TNF naïve patients achieved clinical remission* with Entyvio® vs placebo at Week 52 [2]
The safety profile was consistent between vedolizumab and placebo groups. [2]
Adapted from Feagan BG, et al. Clin Gastroenterol Hepatol. 2017;15:229–239.e5.
Clinical remission at Week 52 (primary endpoint for maintenance therapy): complete Mayo Score ≤2 points and no individual subscore >1 point. [2]
*Exploratory post‑hoc analysis; not powered for statistical comparison.
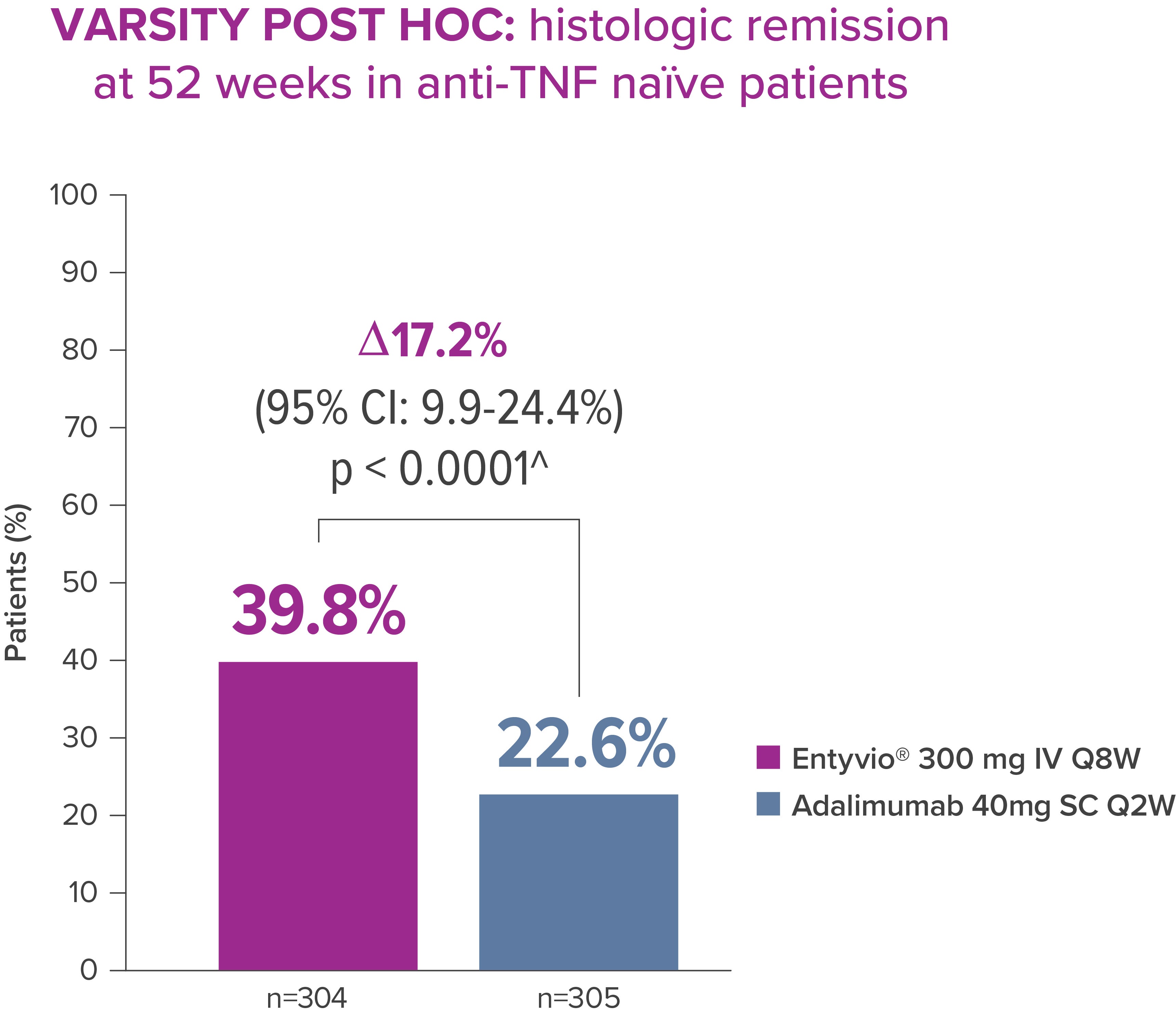
Entyvio® can offer a deeper level of healing for anti-TNF naïve patients, with higher histologic remission* rates than adalimumab [3]
In a post hoc exploratory analysis of the VARSITY trial, 39.8% of anti-TNF naïve patients achieved histologic remission* with Entyvio® vs 22.6% with adalimumab at Week 52 (p<0.0001) [3]
Adverse events occurred in 62.7% of the patients (n=240/383) in the vedolizumab group and in 69.2% (n=267/386) in the adalimumab group. [5]
Adapted from Peyrin-Biroulet L, et al. Gastroenterology. 2021;161:1157–1167.e3.
* Histologic remission (prespecified exploratory endpoint): RHI score ≤2; data from full analysis set, which includes all randomised patients who received ≥1 dose of study drug. [3]
*Histologic remission is not a regulatory endpoint and is not included in the Swiss PI.
*Exploratory post‑hoc analysis; not powered for statistical comparison.
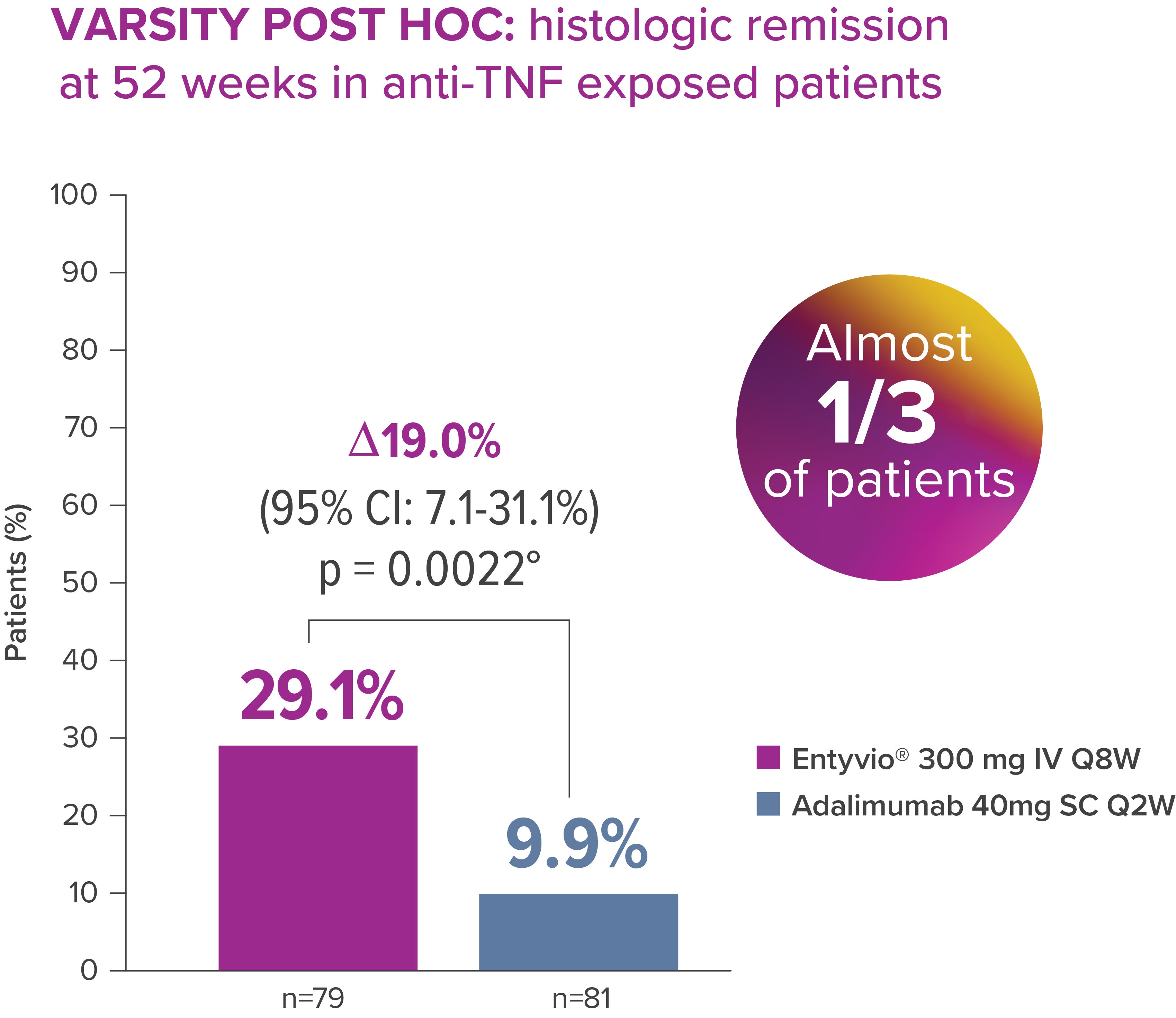
In a VARSITY post hoc analysis of the anti-TNF exposed subgroup of patients, rates of histologic remission* were higher with Entyvio® vs adalimumab at Week 52 [3]
Adverse events occurred in 62.7% of the patients (n=240/383) in the vedolizumab group and in
69.2% (n=267/386) in the adalimumab group. [5]
Adapted from Peyrin-Biroulet L, et al. Gastroenterology. 2021;161:1156–1167.e3.
* Histologic remission (prespecified exploratory endpoint): RHI score ≤2; data from full analysis set, which includes all randomised patients who received ≥1 dose of study drug. [3]
*Exploratory post‑hoc analysis; not powered for statistical comparison.
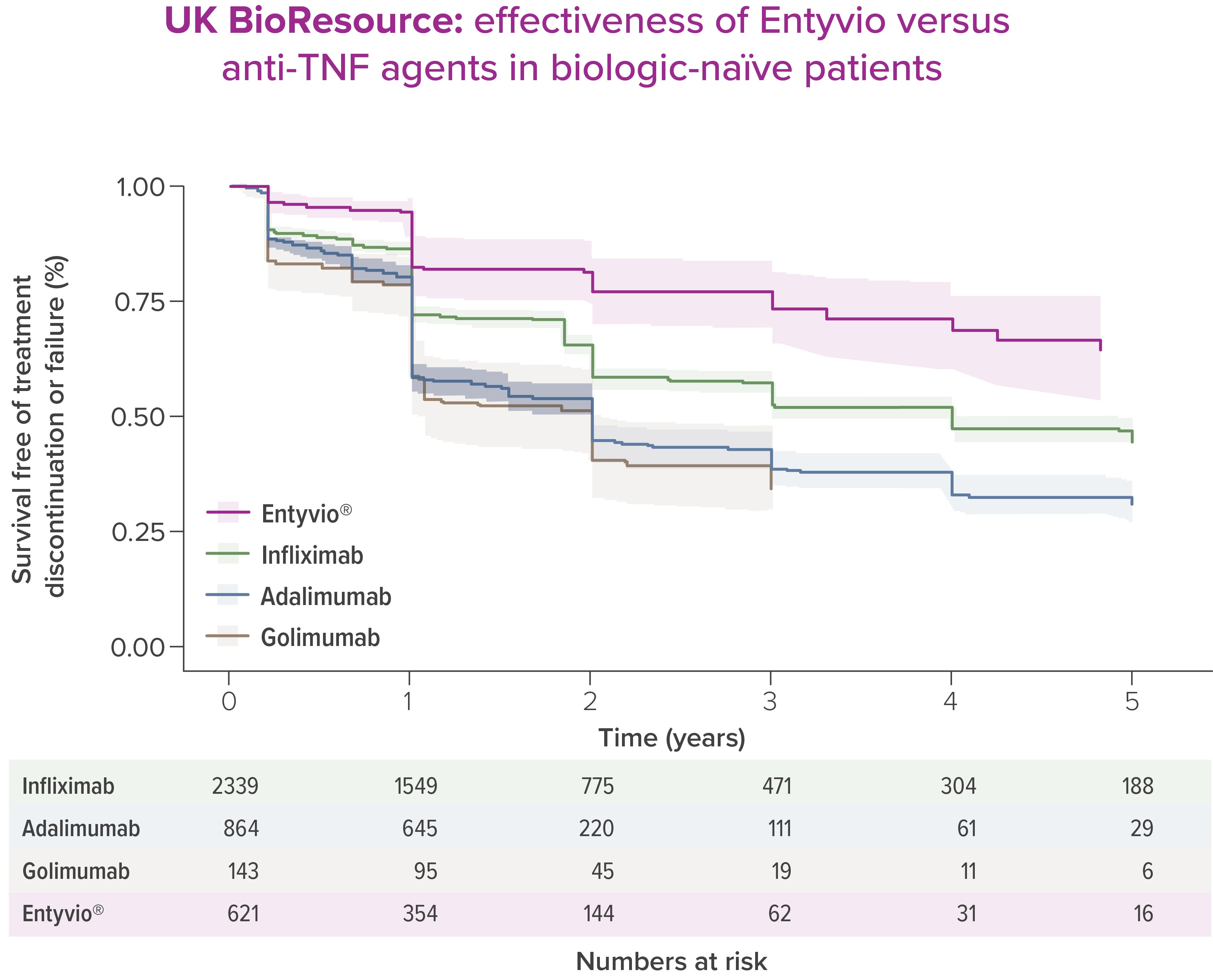
Entyvio® can offer superior long-term effectiveness§ compared with anti-TNFs [4]
In UK IBD BioResource (n=3,967), over 5 years, Entyvio® showed superior effectiveness§ vs anti-TNF agents in biologic-naïve UC patients [4]
Adapted from Kapizioni C, et al. J Crohns Colitis. 2024;18:790–800.
§ Effectiveness of treatment was based on treatment persistence free of treatment discontinuation or treatment failure. Failure was defined as either the occurrence of resectional or defunctioning bowel surgery while on treatment, or clinician coding of treatment failure. [4]
*Real‑world observational data; potential confounders may influence results.
VARSITY was a Phase 3b study that compared the efficacy and safety of Entyvio® with adalimumab in patients with moderately to severely active UC (N=769). A prespecified post hoc exploratory analysis investigated histologic remission, minimal histologic disease activity and composite endoscopic-histologic outcome endpoint based on the Geboes and RHI scoring systems at Week 14 and Week 52. [5]
UK IBD BioResource: A large cohort study compared the effectiveness of biologic therapy (Entyvio®, infliximab, adalimumab, golimumab, ustekinumab) and treatment sequences in patients with UC and CD using real-world data from the UK IBD BioResource (N=13,222). [4]
GEMINI 1 was a Phase 3, randomised double-blind, placebo-controlled study with separate induction and maintenance trials. [5] A post hoc analysis of data from the GEMINI 1 study, collected from 464 anti-TNF naïve patients who received Entyvio® or placebo and 367 anti-TNF exposed patients. In prespecified exploratory and post hoc analyses, the predefined efficacy outcomes from GEMINI 1 were evaluated in the anti TNF-exposed and TNF-naïve ITT populations, respectively. [6]
CI, confidence interval; CRP, C-reactive protein; ECCO, European Crohn’s and Colitis Organisation; IBD, inflammatory bowel disease; IV, intravenous; Q2W, every 2 weeks; Q8W, every 8 weeks; QoL, quality of life; RHI, Robarts Histopathology Index; SC, subcutaneous; STRIDE, Selecting Therapeutic Targets in Inflammatory Bowel Disease; TNF, tumour necrosis factor; UC, ulcerative colitis.