Start Entyvio early to maximize the chance of durable remission and long-term outcomes [1] ‡
Today you met Brad, a 32 year old biologic-naïve patient with moderate-to-severe Ulcerative Colitis. In VARSITY, histological remission at Week 52 was achieved in 25.6% of anti-TNF–naïve patients treated with vedolizumab, compared with 6.7% of those receiving adalimumab5. But let’s start at the beginning.*
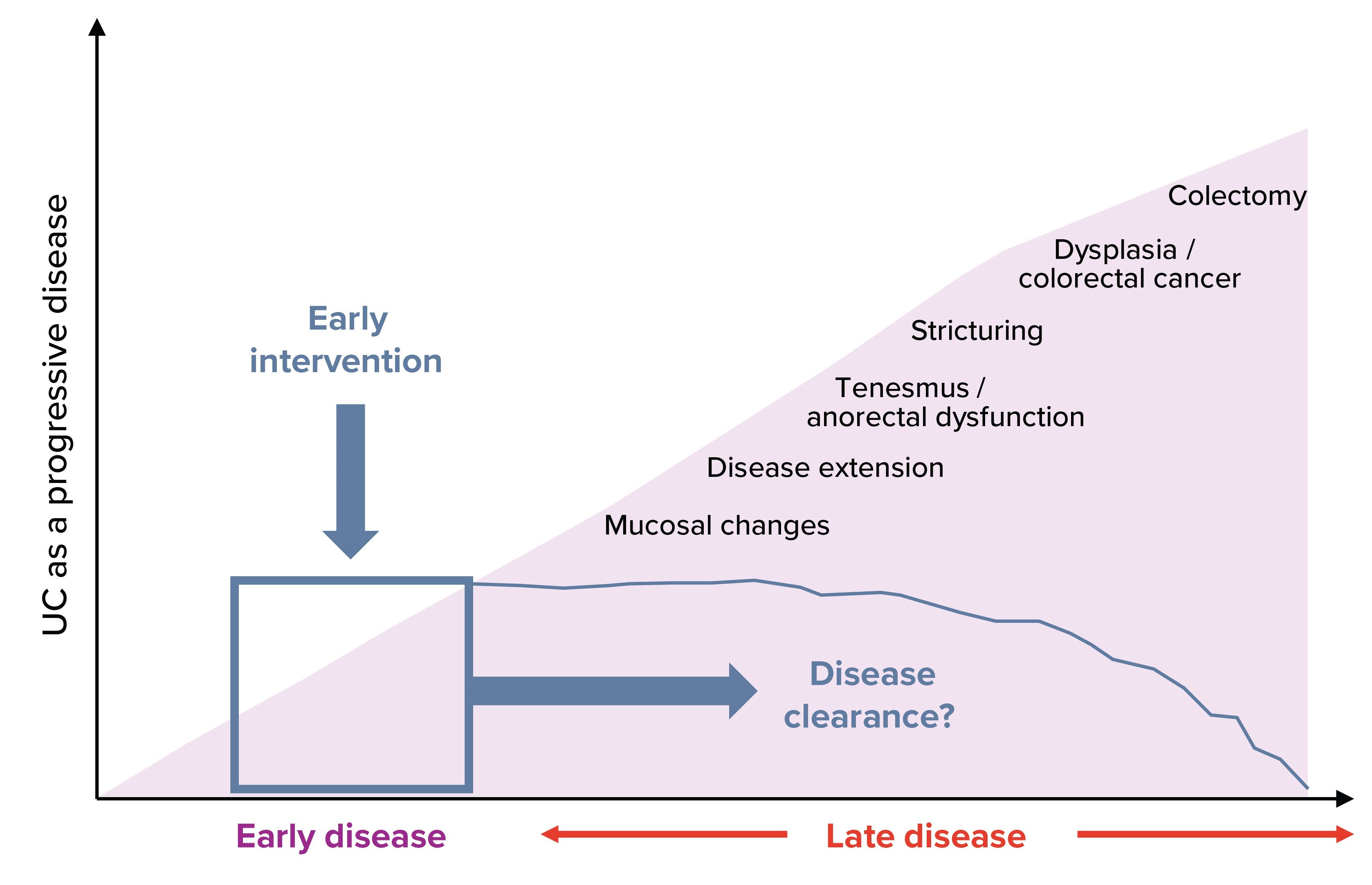
Earlier biologics intervention in appropriate patients with UC, like Brad, may help achieve and sustain remission. Entyvio, with its gut-selective mechanism of action, should be considered early in the treatment pathway when long‑term disease control is a priority. [2]
Adapted from Solitano V. et al. J Clin Med. 2020;9:2646
Early intervention improves long-term outcomes.
Align with STRIDE-II treat-to-target recommendations and ECCO guidelines, which emphasize timely intervention for long-term success. [3] Modifying the natural course of disease is a clear goal for UC management and an early disease intervention could lead to better outcomes. [4] #
Entyvio has shown durable remission in both a randomized controlled clinical trial and a real-world setting in biologic-naïve patients like Brad.
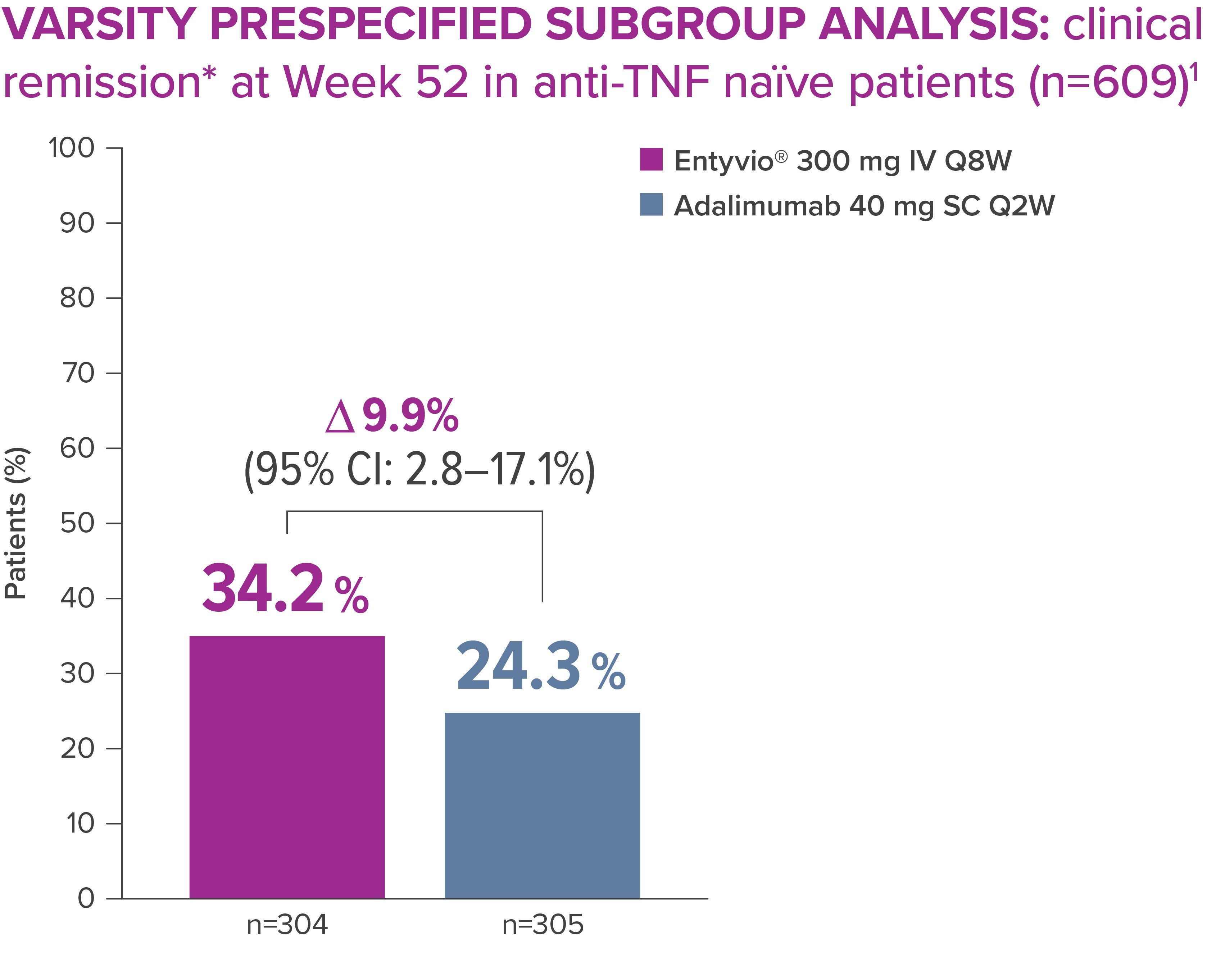
In VARSITY, the first head-to-head trial in IBD, clinical and histological outcomes were directly compared in UC patients treated with Entyvio versus adalimumab. More anti-TNF naïve patients achieved clinical remission* with Entyvio® vs those receiving adalimumab. [5]
Adapted from Sands BE, et al. N Engl J Med. 2019;381:1215–26.
Clinical remission (primary endpoint): total Mayo Score ≤2 points and no individual subscore >1 point on any of the four Mayo scale components; data from full analysis set, which included all randomised patients who received ≥1 dose of study drug. [5]
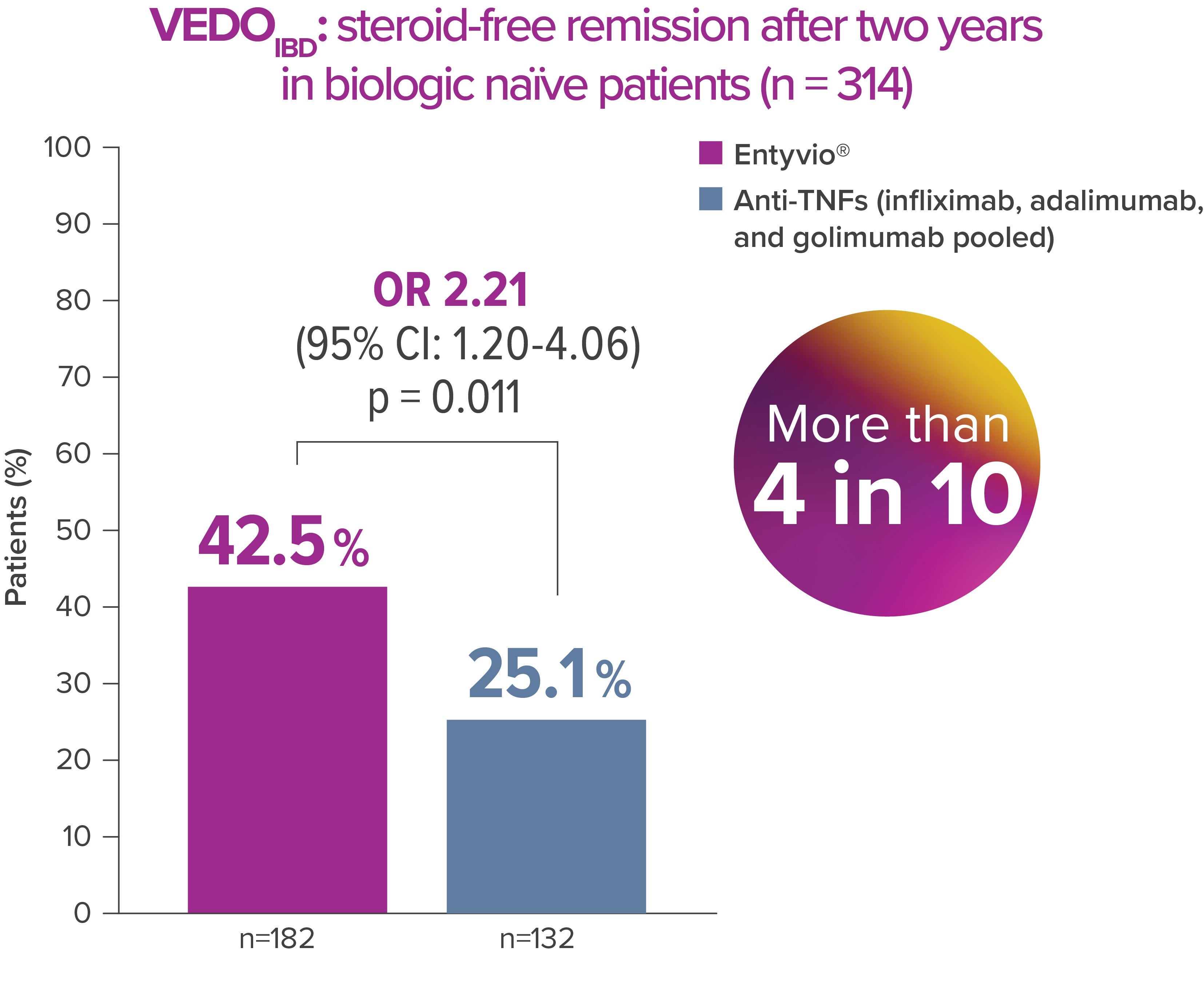
In the prospective, observational real-world study VEDOIBD, compared Entyvio effectiveness with anti-TNF agents. In this study, biologic-naïve UC patients achieved significantly higher 2-year steroid-free remission rates with Entyvio vs anti-TNF agents [6]
Adapted from Bokemeyer B, et al. Aliment Pharmacol Ther. 2023;58:429-442
Steroid-free remission (secondary endpoint): partial Mayo Score of ≤1 plus a bleeding subscore=0 without the use of systemic steroids or oral budesonide in the 8 weeks before the end of induction treatment or during the 6-month period since the last visit during maintenance therapy. [6]
For more information on safety profiles, please refer to the full publication [6], as well as the SmPC.
No relevant differences were found regarding documented adverse events or serious adverse events during treatment with Entyvio® or anti-TNF [6]
VARSITY was a Phase 3b study that compared the efficacy and safety of Entyvio® with adalimumab in patients with moderately to severely active UC (N=769). A prespecified post hoc exploratory analysis investigated histologic remission, minimal histologic disease activity and composite endoscopic-histologic outcome endpoint based on the Geboes and RHI scoring systems at Week 14 and Week 52. [5]
VEDOIBD was an observational real-world evidence study based on prospectively collected data from the VEDOIBD registry (N=314). The aim was to compare the effectiveness of Entyvio® vs anti-TNF therapies in biologic-naïve UC patients. The primary endpoint was clinical remission at the end of induction (Week 14) and during maintenance treatment at 1 and 2 years. Secondary endpoints were clinical response and steroid-free remission at Week 14 and 1 and 2 years. [6]
CD, Crohn’s disease; CI, confidence interval; IBD, inflammatory bowel disease; OR, odds ratio; SMD, standardised mean difference; TNF, tumour necrosis factor; UC, ulcerative colitis;
* Exploratory post‑hoc analysis; not powered for statistical comparison.
# Disease clearance is an exploratory, non‑regulatory composite endpoint not included in the approved Swiss PI.
‡ Long-term success in IBD is defined as a change in the disease course (reducing hospitalisations, surgeries, bowel damage and disability). [1], [7]