Disease clearance matters, make it part of your treatment goals with Entyvio.
Disease Clearance is a composite endpoint combining clinical, endoscopic, and histologic remission, a composite endpoint linked to better long-term outcomes. [1]
Disease clearance therefore goes beyond symptom control and visible parameters, by combining resolving of symptoms, visual normalization of the mucosa and normalization of the microscopic features of the disease. [1]
An IOIBD-proposed definition of disease clearance
Disease clearance is emerging as a potential target in the treatment of UC [1] and consists of three separate endpoints. Definitions vary slightly between studies, but the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD)-proposed definition is as follows:
*Disease clearance is an exploratory, non‑regulatory composite endpoint not included in the approved Swiss PI.
In the VARSITY trial, ENTYVIO achieved higher rates of disease clearance compared to adalimumab. [2]
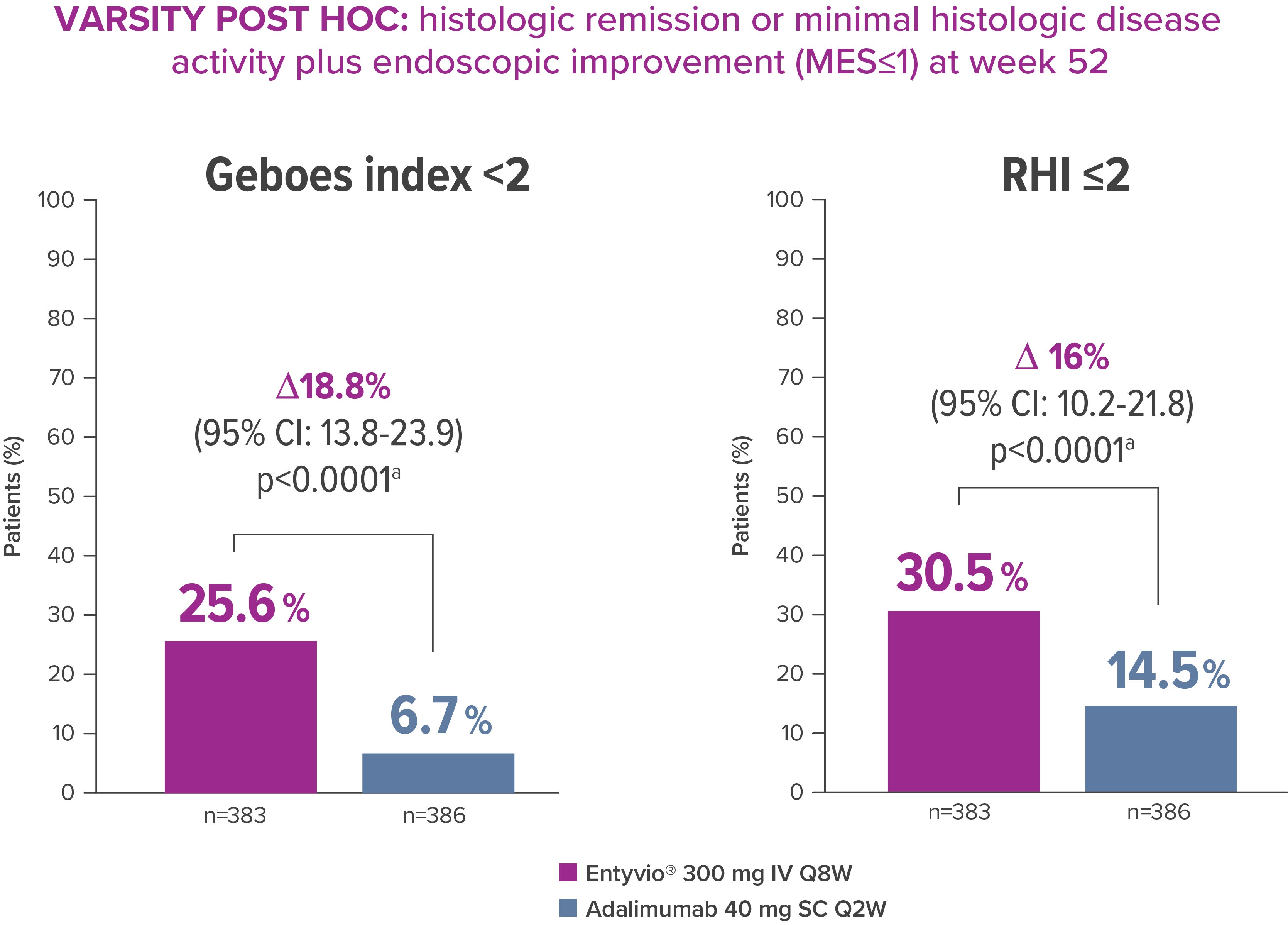
In a post-hoc analysis of VARSITY, the first head-to-head trial in IBD, more than twice as many patients treated with Entyvio achieved histologic remission plus endoscopic improvement than those treated with adalimumab [3]
VARSITY POST HOC: explores the associations between histologic outcomes and endoscopic improvement (n=769 patients with UC) [3]
a, nominal P values
CI, confidence interval; IV, intravenous MES, Mayo endoscopic subscore; RHI, Robarts Histopathology Index; SC, subcutaneous
*Exploratory post‑hoc analysis; not powered for statistical comparison.
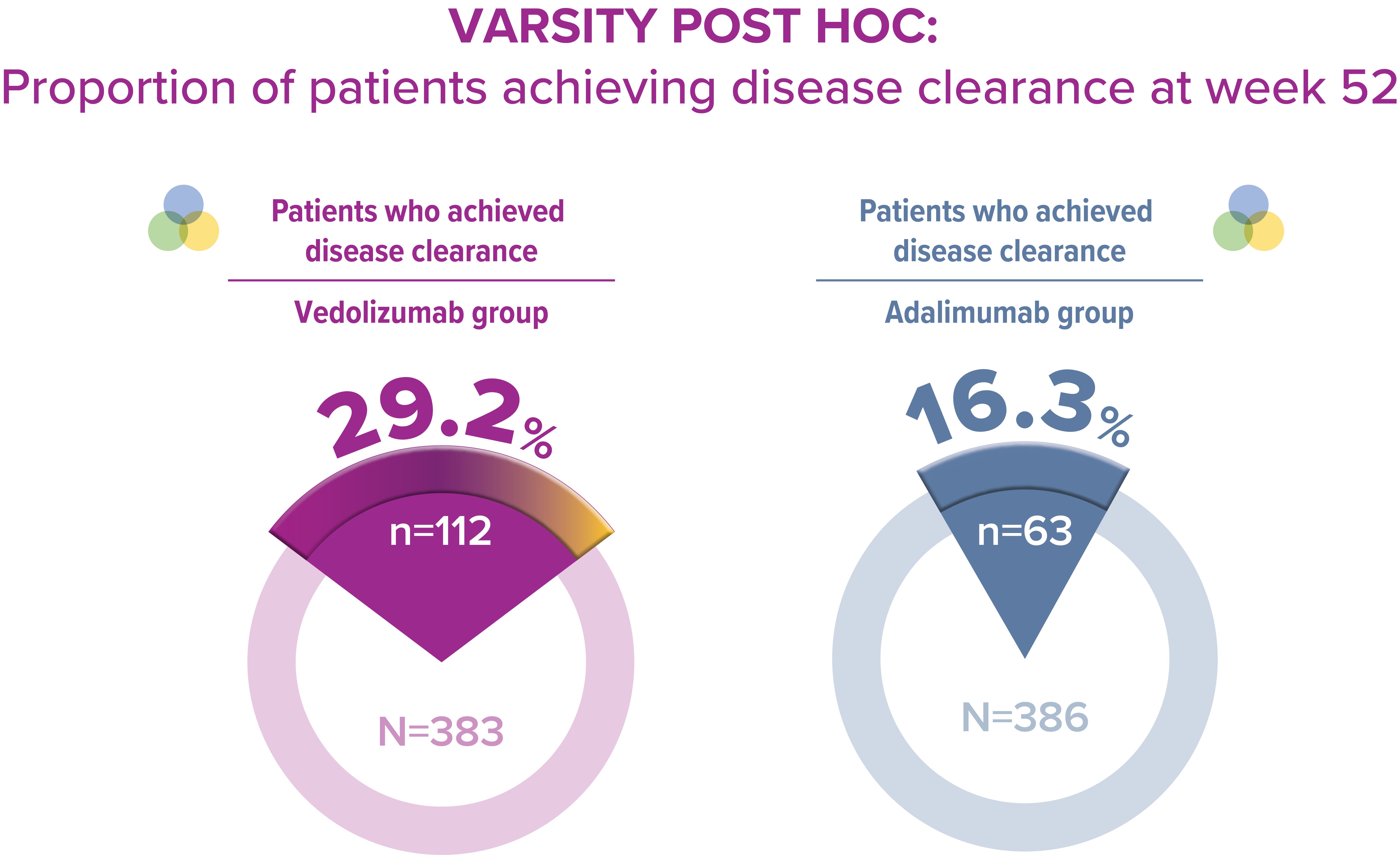
In another analysis of the VARSITY trial data, almost twice as many patients treated with VDZ achieved disease clearance than those treated with ADA [2]
Proportion of patient achieving disease clearance at week 52 is a novel and attainable composite outcome, achieved by nearly one third of patients treated with VDZ in the VARSITY study [2]
*Exploratory post‑hoc analysis; not powered for statistical comparison.
Emerging paradigm: The VERDICT study reinforces disease clearance as an achievable key target in UC management. [1]
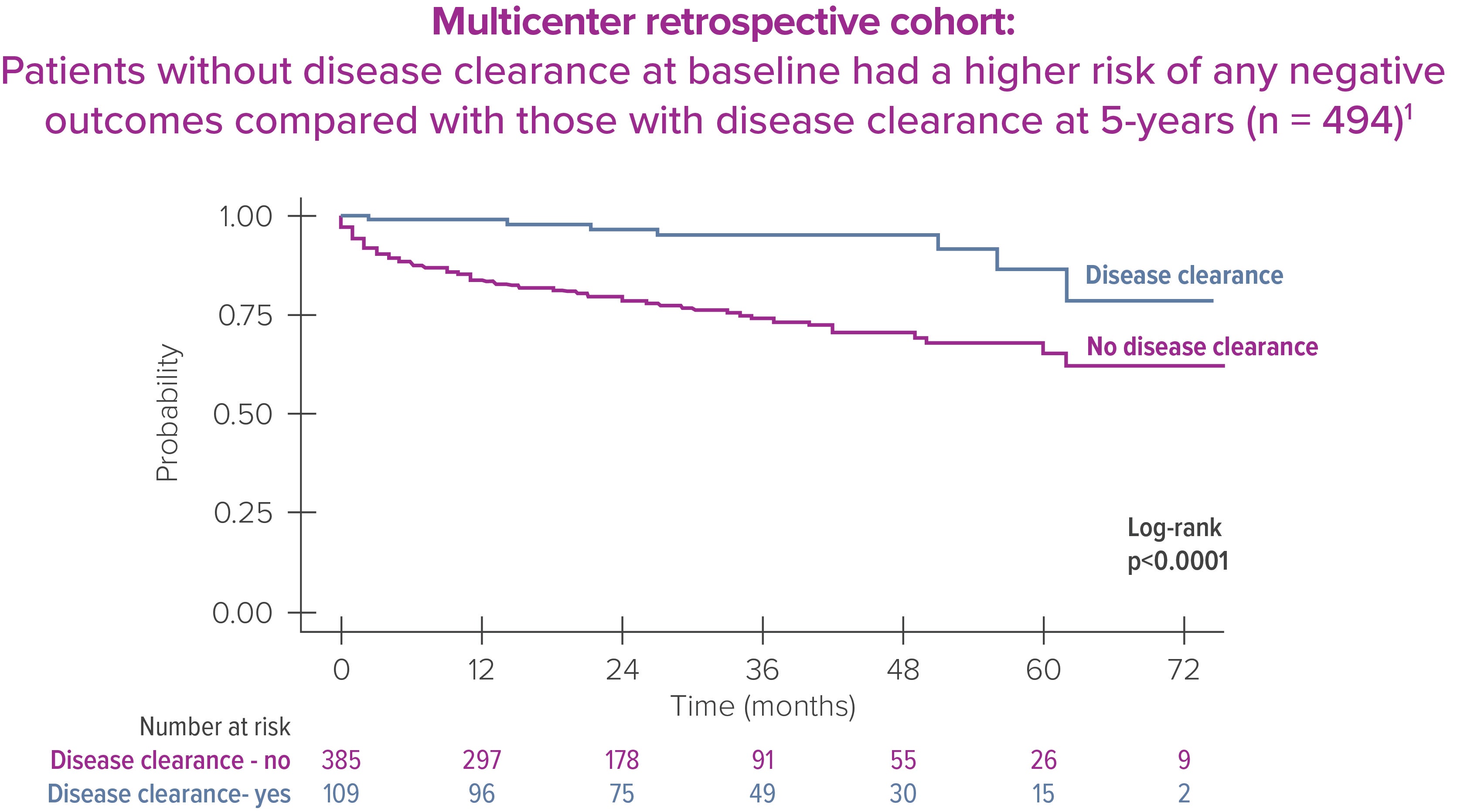
Early disease clearance in UC patients is associated with lower risk of hospitalization and surgery in a retrospective cohort study. [1]
Disease clearance in UC was defined as simultaneous clinical (partial-Mayo score ≤2), endoscopic (endoscopic-Mayo score = 0), and histological (Nancy index = 0) remission [1]
Disease clearance is key for patients with IBD because it can reduce the risk of hospitalization by 80% (p<0.001) and the risk of surgery by 86% (p=0.007) and lower overall healthcare costs [1]
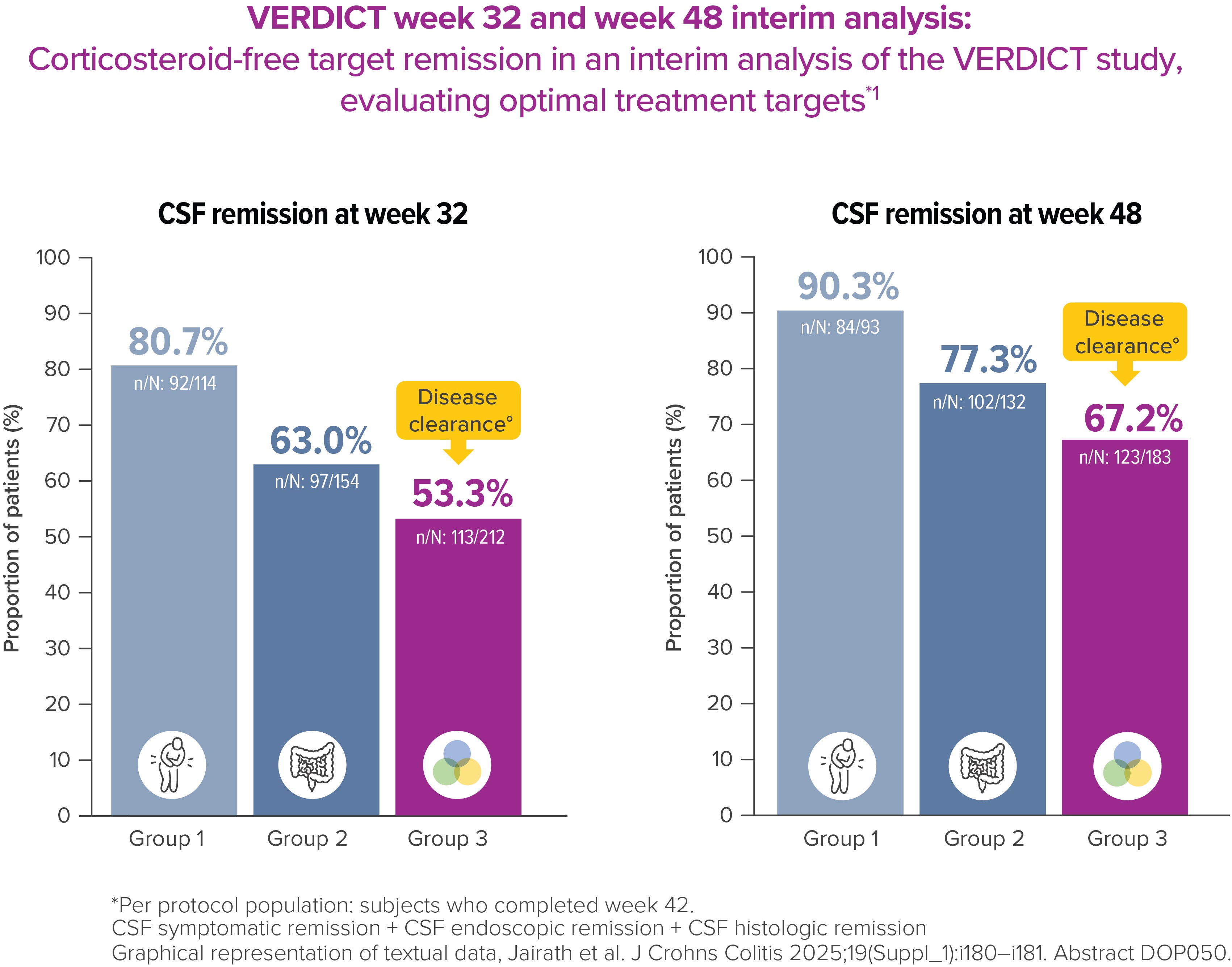
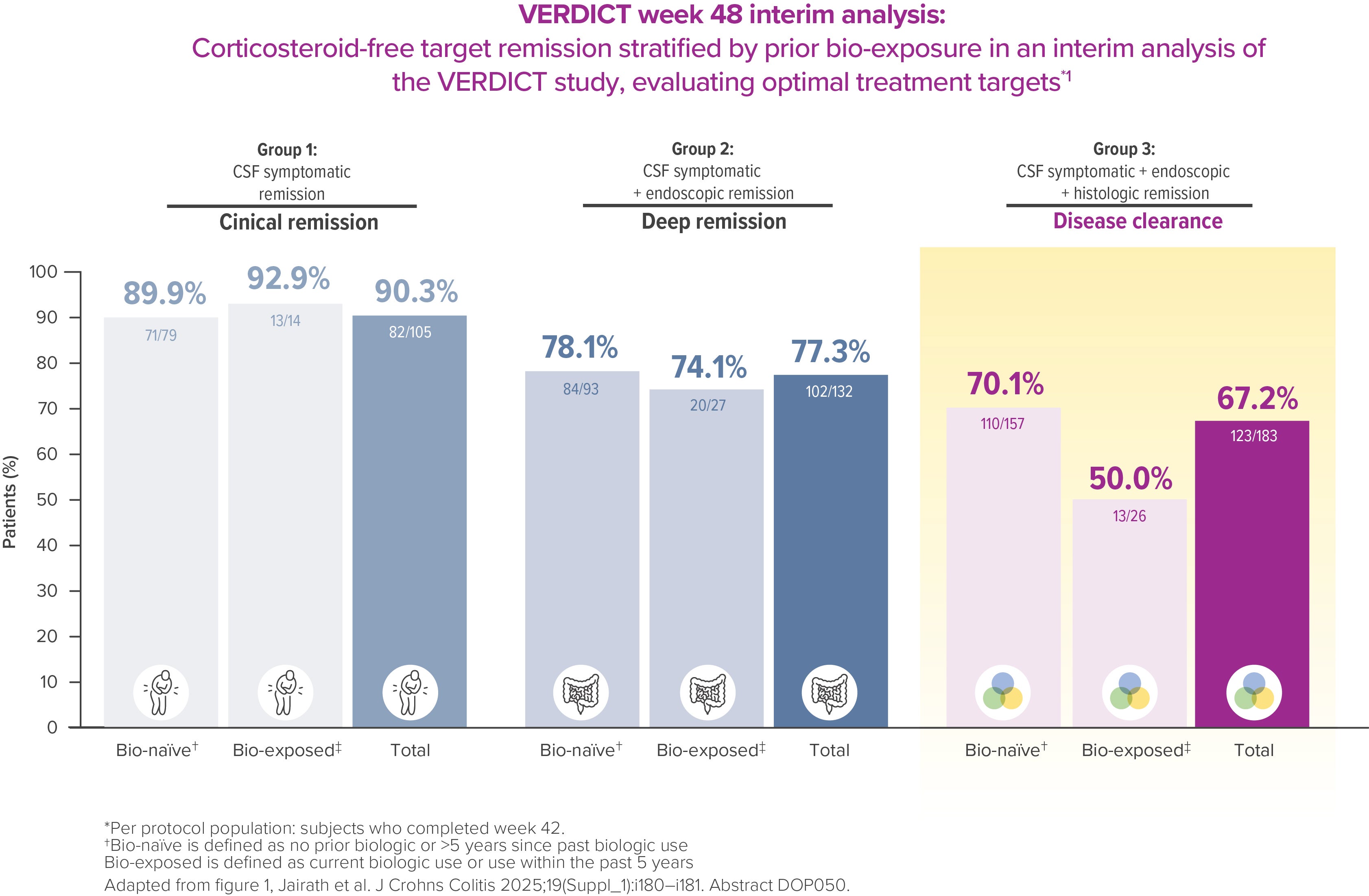
VERDICT is the first randomized controlled trial investigating the optimal treatment target for UC patients. Interim data showed that corticosteroid-free disease clearance is achievable in 67% of patients by week 48, with an improvement vs week 32 [4] *
Per protocol population: subjects who completed week 42.1
°CSF symptomatic remission + CSF endoscopic remission + CSF histologic remission [4]
In VERDICT, a higher proportion of bio-naïve patients achieved corticosteroid-free disease clearance at week 48 compared to bio-exposed patients [5]
Per protocol population: subjects who completed week 42.1
Bio-naive is defined as no prior biologic or >5 years since past biologic use, bio-exposed is defined as current biologic use or use within the past 5 years [4]
Once clearance is achieved, what could be next for Brad?
Let’s talk about long-term success and sustained outcomes in the next and final section.
VARSITY was a Phase 3b study that compared the efficacy and safety of Entyvio® with adalimumab in patients with moderately to severely active UC (N=769). [5] †‡ A prespecified post hoc exploratory analysis investigated histologic remission,* minimal histologic disease activity and composite endoscopic-histologic outcome endpoint based on the Geboes and RHI scoring systems at Week 14 and Week 52. [3]
The VERDICT study is a 96‑week trial in which patients with moderate to severe ulcerative colitis were treated with Entyvio® and randomized into three different treatment target groups: corticosteroid-free symptomatic remission (Group 1; n=132), corticosteroid-free symptomatic and endoscopic remission (Group 2; n=198), or corticosteroid-free symptomatic, endoscopic, and histological remission (Group 3; n=330). The objective of the study is to determine the optimal treatment target, as measured by the time from achievement of the target to the occurrence of a disease-related complication.
Abbreviations: CSF, corticosteroid-free remission; IOIBD, International Organization for the Study of Inflammatory Bowel Diseases; MES, Mayo endoscopic subscore; RHI, Robart’s histopathology index; UC, ulcerative colitis